Joseph Nicéphore Niépce is generally acknowledged as the first successful photographer who in 1826 or 1827 used his heliography process to capture an image that remained fixed. This process was however limited by the extremely long exposure time required by the materials involved. Shortly after this achievement, he was introduced to French artist and businessman Louis Jacques Mande Daguerre (1787-1851) through their optician, Charles Louis Chevalier, who manufactured the lenses for their Camera Obscura. Together they strove to improve the process Niépce had fathered.
Daguerre at this time was a Romantic painter and printmaker best known for his Diorama, a popular attraction in Paris employing theatrical painting and lighting effects in a 360-degree display that created an illusion of movement, such as changing seasons, a train crash, or a volcano eruption. His painted theatrical gauze had images on both sides and by utilising both backlighting and toplighting, one picture would dissolve into another successfully creating a diorama. He employed a Camera Obscura as a drafting aid for the creation of this illusion, but wanted a quicker way to produce scenes than hand painting them with the meticulous detail required to create the effect.
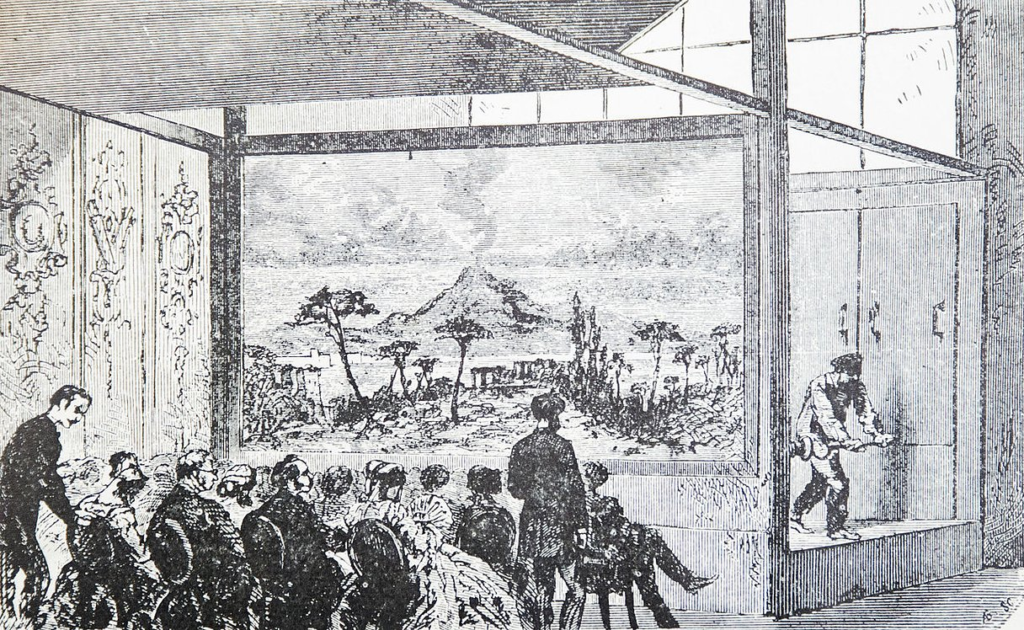 An illustration of people watching Daguerre’s diorama
An illustration of people watching Daguerre’s diorama
Learning of Niépce’s work, Daguerre eventually joined forces with him in 1829. In the course of their partnership, Niépce and Daguerre switched from pewter to Sheffield plates (a fusion of copper and silver), which were produced as a standard hardware item by heating and rolling silver foil in contact with a copper backing. They then innovated by fuming iodine onto the bitumen and silvered plates to yield positive silver iodine images of greater detail. Daguerre soon discovered that the silver iodine itself was light sensitive and could be used without the bitumen to create images. He also discovered (allegedly though the accident of breaking a mercury thermometer stored with some exposed plates) that a latent image on the silver plates could be revealed by exposure to mercury fumes. However, much like other experimenters in the field were finding, he could not control the continued darkening of the image with its subsequent exposure to light; the silver iodine layer remained light sensitive. Daguerre struggled with this problem until 1837, when he tried saturating the developed image in hot salt water. This procedure cleared the unexposed silver iodine from the image, making it now light-fast, or “fixing” the image. Two years later, following the advice of Sir John Herschel, he adopted hyposulfate of soda (now thiosulfate of soda), or “hypo”, as the fixing solution.
In 1833, before Daguerre had actually made these discoveries and improvements to the process, Niépce suffered a stroke and died. Daguerre believed that his subsequent changes were so significantly different from Niépce’s earlier work that his improvements constituted a completely new process, whereupon he named his new images daguerreotypes.
 “Still Life” taken by Louis-Jacques-Mandé Daguerre (1837)
“Still Life” taken by Louis-Jacques-Mandé Daguerre (1837)
By 1837 he had produced the still life above. It was a remarkable achievement that within a decade the process had been improved to such a degree that we have a completely acceptable picture with remarkable detail.
Daguerre now had a viable process for capturing images, and sought a way to profit from his discovery. He initially attempted to sell the process for a lump sum in 1838, and then via a subscription scheme. When this proved unsuccessful he adopted a more political approach. Enlisting the aid of Francois Arago, an influential member of the French Academy of Sciences, Daguerre was able to present the daguerreotype to the French government as “indispensable that the Government should compensate M. Daguerre directly, and that France should then nobly give to the whole world this discovery which could contribute so much to the progress of art and science.” This effort evidently proved convincing, as in 1839 Daguerre and Niépce’s son were granted an annuity by the government for this achievement. This was passed by both houses, and the King of France signed the bill and directed that the invention should be disclosed to the public on 19th August 1839. Daguerre soon published a manual of his process and this early form of “open source” technology led to an explosion of interest in the daguerreotype process throughout Europe and the United States.
The first daguerreotypes used “slow” Chevalier lenses, and the silver iodine layer had a light sensitivity equal to a modern rating of 0.001-0.005 ISO, leading to long exposure times of up to one hour. Portrait photography was therefore impossible, and the first daguerreotypes focussed on subjects like still life, street scenes and architecture. Over time exposure times were reduced significantly as the chemical processes were refined and faster lenses were invented.
By 1841, Chevalier in France, Ross in London, and Petzval in Austria began to develop better achromatic (non-distorting) lenses, featuring larger effective apertures, for express use in daguerreotype cameras. The use of bromine and chlorine fumes to form light-sensitive silver bromide and/or silver chloride was also adopted, yielding plates with greater light sensitivity. The use of smaller cameras and plates also allowed the available light to be more effectively concentrated, resulting in shorter required exposure times. By 1842 exposure times had been reliably reduced to under one minute.
The final image made in a daguerreotype was a very fragile direct positive image that could be easily smudged. It had a polished surface finish like a mirror, requiring the plate to be angled to view the image properly. The highlights were created by greater amounts of silver amalgam particles on the plate surface and the shadows of the image were created by few or no silver amalgam particle deposits, allowing the polished silver finish to absorb light, thus appearing dark. The viewer’s own reflection can be seen in the image at times, and depending on the angle of viewing and the ambient lighting, the image could provide the illusion of being a negative instead.
The fragility of the plate required protective glass-covered cases or frames to be provided for finished daguerreotypes. Another characteristic of the daguerreotype is that the image is reversed with writing appearing backwards. The image was also a unique original that could not be projected or enlarged, or reproduced directly. The only way to get a copy of a daguerreotype was to take another daguerreotype of the first finished plate.
The daguerreotype was capable of extremely fine detail and image resolution. Magnified viewing of well-focused vintage daguerreotypes can resolve minute features with no loss of detail. In the process of restoring the Charles Fontayne and William Porter 1848 daguerreotypes of the Cincinnati waterfront, the George Eastman House conservators estimated the resolution of the 6.5 x 8.5-inch plates as the equivalent of 140,000 megapixels each; the resulting panorama could be blown up to 170 by 20 feet without losing clarity.
The daguerreotype process took the world by storm, but particularly so in the United States; the process was free, without patent restrictions or licensing fees. Daguerre’s instruction manuals provided followers with the needed technical foundation to pursue their own endeavors. Within a year of the process’s release to the public, improvements in cameras, lenses, and chemistry made portraiture possible, which in turn spawned an entire daguerreotype portrait industry. While still not inexpensive, for US$2 to US$5 almost anyone could get a portrait made in almost any major city. By 1853, it was estimated that 3 million daguerreotypes per year were being made in the United States.
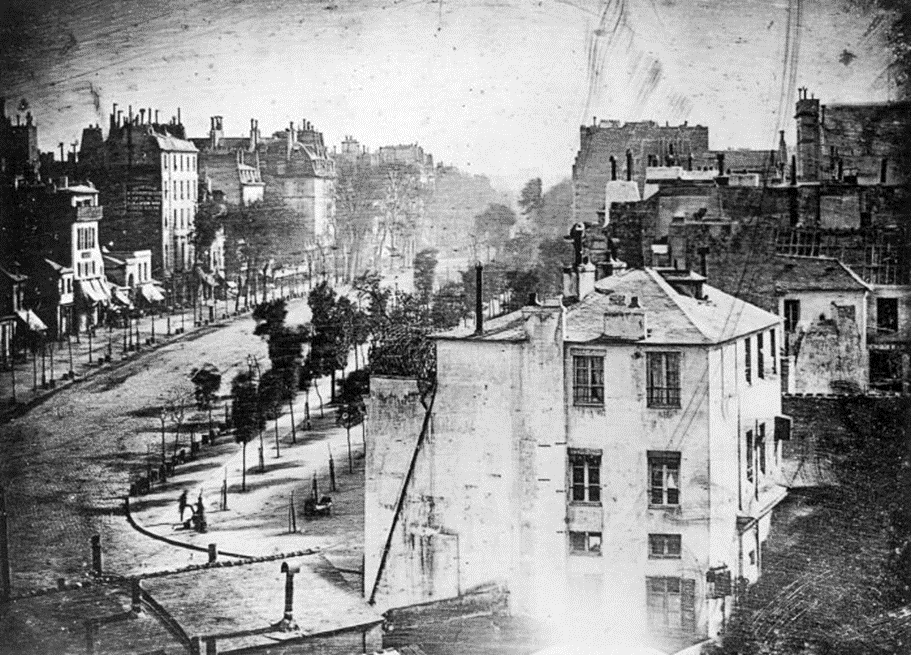 “Boulevard du Temple“ taken by Daguerre in Paris (1838), widely considered to be the first photograph to include an image of a human and the first ever street photo.
“Boulevard du Temple“ taken by Daguerre in Paris (1838), widely considered to be the first photograph to include an image of a human and the first ever street photo.
